A few weeks ago, one of our portfolio companies Kleiner Device Labs (“KDL”) announced they’d officially received FDA 510(k) Clearance for their KG™2 Surge™ Flow-Thru Interbody System (“KG2”).
This means the KG2 is now “cleared” to be marketed, sold, and otherwise used in the operating theatre.
However, before the team is ready for a full-scale launch of the product, they’re going to run a small-scale roll out to a select group of surgeons.
In the medical device industry, this is known as an “Alpha Launch”… and it’s a key step in the company’s path towards full commercialization of this new technology.
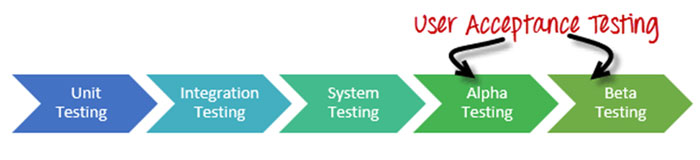
If you are familiar with the software industry, “Alpha Testing” and “Beta Testing” refer to a type of User Acceptance Testing.
Broadly speaking, Alpha Testing is mainly performed by the in-house software team while Beta Testing is done by potential users or customers.
However, in the world of consumer and enterprise software, the mandate is often to “move fast and break things.”
Speed to market is key in the tech world. Even if there are bugs in the software, it’s generally more important to ship software as fast as possible to the end user and (hopefully) fix problems with continual updates.
The commonly held understanding is that the consumer will tolerate failures so long as they have access to the latest and greatest technology.
However, in the much higher stakes world of medicine, this attitude simply doesn’t work.
Risk management is a far greater consideration in the development cycle as efficacy, stability and predictability are key.
Unlike Alpha Testing in software, medical devices must be a “finished product” in order to earn 510(k) Clearance.
However, similar to software, the device still needs to be used in the operating theatre under real world conditions.
“Alpha Surgeons” represent an initial group who will be the first to use a new device in live patient procedures.
KDL has hand-picked a small team of orthopedic spine & neuro-surgeons who represent a cross section of surgical theaters, from hospitals to surgery centers.
Several of these surgeons participated in a cadaver testing laboratory exercise last April. This means they already have some familiarity with the device.
The first surgeries done by this group will document procedure processes and results, which will be presented to surgeons more broadly in the U.S., and will provide important proof points for the industry and the insurers who predominantly pay for the spinal fusion procedures for which the KG2 is specifically designed.
For all of our Equifund members who have invested – or are planning on investing – the Alpha Launch marks a critical inflection point in the life cycle of the KG2.
The same goes for other medtech companies you may be looking at. Keep that in mind when investing (especially in early-stage deals).
Sincerely,
Jake Hoffberg – Publisher
Equifund